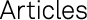
Max Planck Institute of Colloids and Interfaces, Potsdam, Germany
Atelier Saralya, Kotzen, Germany
TUM School of Life Sciences, Freising, Germany
This article appears in West 86th Vol. 31 No. 1 / Spring–Summer 2024
This article focuses on the material properties of tree bark, its cultural history, and its potential for future applications. It discusses the use of bark ash in ceramic glazing with the aim of promoting sustainable material utilization and revaluing what is commonly regarded as waste. Historically, craftsmanship without wasting resources was a symbol of superior quality and economic viability. With industrialization, tree bark became an industrial by-product. Through experimental investigations, this study explores the chemical composition of selected bark ashes and their potential as an additive for ceramic glazing. Combining craftsmanship, design, and scientific methods, the study showcases the possibilities of bark ash as a glazing ingredient, producing a range of colors and surfaces. In conclusion, this study shows the integration of interdisciplinary knowledge—from sciences, humanities, history, and crafts—to foster a holistic approach toward sustainable material utilization and redefine the significance of what is currently deemed waste.
Introduction
The evolution of human culture is closely intertwined with craftsmanship, its techniques, and the choice of materials. For many centuries, the resources that humans used for making tools, clothing, and everyday items were of invaluable worth. Craftsmanship that wasted no material was regarded as a sign of high quality and virtue. Everything derived from a tree, animal, or stone was used. However, with the advent of industrialization, our relationship with materials changed. Efficiency and production rates became the main drivers. Materials like tree bark lost their importance and cultural value, turning into a by-product of the timber industry.1 In times of climate change and limited resources, rethinking our use of materials is essential.
In this paper, we argue that the use of tree bark is deeply connected with human civilization and its relationship with the environment. A research approach that combines craftsmanship, cultural history, and natural science to address contemporary industrial design questions can pave the way for a sustainable economy where all biomaterials are perceived as valuable resources rather than waste.
Here, we provide a summary of the existing relationship between man-made mass (waste) and the natural biomass on earth, exploring its origins and manifestations in the wood processing industry. This industry serves as a representative example of the contemporary approach to utilizing renewable biobased resources. We will then examine how the applications of tree bark have evolved before and after industrialization. Bark ash emerges as the ultimate waste of industrial material processing in this context. We will summarize the history, processing methods, and properties of bark ashes before describing and analyzing our own initial experiments to create porcelain glazes that contain bark ash.2 These results undergo both visual and chemical analysis. We conclude these “proof of concept” experiments by discussing the potential of tree bark ash for future ceramic design applications. Within the scope of this article, bark is showcased as a material that can transition from an industrial by-product to a sustainable resource, reminiscent in its methods of the earliest utilizations of plant materials for crafting artifacts.
This study demonstrates how a holistic research approach, combining craftsmanship, design, and scientific methods, can guide a material from the past into the future.
Times of Waste
In the twenty-first century, human activities and the accumulation of human-made objects have profoundly impacted Earth like never before. Although humans make up only about 0.01 percent of global biomass, our activities have had a significant and diverse impact on the planet for the past three thousand years.3 Around 1900 anthropogenic mass accounted for approximately 3 percent of global biomass. In 2020 anthropogenic mass surpassed overall biomass worldwide, with continuous increases signaling the drastic changes human societies underwent in the twentieth century.4
Reasons for this can be illustrated, for example, by the history of industrial production in the United States. The US economy boomed after World War I because of rapid modernization, with significant growth in the automotive, electric, and chemical industries.5 This growth was achieved by innovations in production methods, particularly attributed to Frederick Taylor and implemented by Henry Ford, enabling mass production and consumption.6 The 1950s were characterized by optimism and the widespread use of synthetic materials. These materials, however, often required energy-intensive processes and toxic components, resulting in nonbiodegradable products with lasting environmental impacts. Without a change in the prevailing direction, the total man-made mass could triple Earth’s dry biomass by 2040.7
How can we intervene? A promising contribution is the use of renewable resources. The material that probably first comes to mind is wood. In 2020 alone, around 80 million cubic meters of wood were harvested in Germany despite the suffering of forests from a changing climate.8 Drought and heat are damaging forests and increasing stressors—for instance, the spread of pests like the bark beetle. A more resource-oriented approach is to use as much of the harvested tree as possible in high-quality and long-lasting ways. Unfortunately, the reality is different: about 44 percent of the wood taken from the German forests is burned without further use—more than half of it as energy wood, the rest as sawmill by-products.9 Moreover, wood is utilized for low-value applications such as particleboards, fiber materials, or paper production, which significantly shorten the usage cycle of the harvested wood.10
One by-product of the timber industry is tree bark, accounting for 9–21 percent of a tree’s volume.11 When a tree is felled for industrial purposes, the bark mostly becomes waste. German sawmills produce about 4 million cubic meters of bark annually.12 Only a small portion of this bark is further processed. The majority is wet-burned in sawmills.13
The Utility of Tree Bark in History
The current handling of bark as described in the previous section presents a strong contrast to its long history of human use: tree bark was prized as a by-product of wood to maximize the use of the entire tree trunk. Tree bark has been valued for its extracts probably since the Pleistocene era, with archaeological evidence indicating the production of adhesive tar from birch bark by Neanderthals.14 Another notable example is the production of leather through tanning animal skins using the tannins naturally found in tree bark.15
In addition to substance extraction, tree bark was also used as a raw material in different regions of the world; for example, for textiles and as a construction material for dwellings and means of transport. Charlett Wenig and coauthors describe a range of examples:
The finding of an approximately eight-thousand-year-old bark cloth beater in southern China is one of the earliest pieces of evidence for bark cloth making. Early bark cloth is non-woven and made by beating the plant fibres after retrieving the inner bark. With human migration, the knowledge as well as the plants (e.g. paper mulberry) expanded to various regions. Bark cloth is still made in islands of the Oceania as “tapa” as well as in Central America and Uganda. However, the inner bark material was also spun into cord or yarn and used for tying and knotting. This bast-like material could be knitted, woven and braided into two- and three-dimensional objects—bags knitted from bark yarn have been found in Australia, for example, and mats woven and braided from birch bark (from useful underlays to coverlets diligently worked with quills with feathers), boxes (from salt vessels to bags) and useful helpers such as the pot scourer (a ball made from rolled-up strips of bark) but also bark scrolls with drawings on it have been found in the Finnish/Russian region of Karelia.16
Since the Neolithic Age, bark was utilized for a variety of construction purposes such as roofing and flooring. Outer bark was recognized as a valuable and practical material for centuries; Indigenous peoples in North America, for example, crafted their canoes using birch bark.17 In Austria, loggers traditionally used bark to build huts in the forest.18 The wide use of bark in Europe continued until the beginning of the twentieth century. Today, it is a supplementary or craft material, limited to certain applications such as basket weaving, leather tanning, coloring, and various chemical processes.
Uses of Tree Bark in Modern Industry
The diminished importance of tree bark became apparent by the mid-twentieth century. Industrialization and globalization led to the concentration of wood processing in certain locations worldwide. From the twentieth century onward, vast amounts of timber were harvested from regions such as Scandinavia, Russia, and North America, and then shipped to remote sawmills for additional refinement.19
The procedure of removing bark is typically centralized at the sawmill, just before the wood undergoes further processing. The high output in sawmills and related wood industries results in the rapid accumulation of immense volumes of bark.20 Today, the commercial uses of bark fall into three basic categories: as a source for horticultural materials when shredded into small pieces; as an extraction source for biomedical applications and adhesives; and as a fuel source through combustion.21
Side products like bark have often been assigned less economic importance and are subjected to less extensive research than wood. This devaluation can be attributed to the more complex and variable structure of bark, both within and between different species, as compared to wood. Bark’s high degree of variability results from continual changes due to cell divisions and growth between the primary and secondary tissue, also known as the phloem.22 However, as we face the ongoing challenges of climate change and scarcity of resources, the need for more comprehensive understanding and utilization of all parts of a tree, including the bark, is becoming increasingly apparent.
Bark Ash and Its Composition
It is essential to delve deeper into the properties of bark and its potential applications. Bark is mostly burned moist and contains less volatile matter and a higher percentage of fixed carbon than wood.23 Both of these characteristics tend to lower bark’s heating values.24 Ash quantities of more than 10 percent by weight have been reported for combustion residues of some hardwood barks, whereas for wood, these values are typically below 1 percent by weight.25 Ash is produced when plant material undergoes oxidation in fires beyond charring, to a stage where most of its oxygen, hydrogen, and carbon have burned and evaporated completely. Trace elements and other mineral constituents then dominate the remaining material.26
The main element found in bark ash in the mineral fraction is calcium, with 82–95 percent, followed by potassium and magnesium.27 Other metal oxides and some other elements (Na2O, Fe2O3, P2O5, etc.) are present to a small extent, typically less than 1 percent.28
The chemical composition of bark is not only species dependent but also a result of external factors such as the chemical composition of the soil. Hence, the chemical composition of each tree can be different. High variability of biomaterials is well-known and in industrial contexts is often seen as a problem: it makes their use on a larger scale more challenging. However, the use of bark ash as an additive for concrete production or as a supplementary material in horticulture was investigated in the past.29
Historic Uses of Plant Ash
The history of plant-ash use shows that despite its high chemical variability, plant ash is a material with potential. Ash has always been an important additive in ceramics, glass, and glazing crafts throughout human history. Plant ashes have been crucial in the historical development of glassmaking. Relevant techniques likely emerged in Mesopotamia during the late Bronze Age before being transmitted to Egypt.30 In contrast to the natron-type composition of Roman and late antique glasses in Egypt and Greater Syria, Mesopotamian glass utilized soda-rich plant ash, specifically from halophytic plants like Salicornia kali and Salsola.31 Over the centuries, a variety of plants have been used for glassmaking, including not only halophytes but also the beech tree, whose wood ash played a role in glass production from late antiquity.32
The utilization of plant ashes in pottery glaze pre-dates these practices, reaching back even earlier in history.33 The history of ash glaze—ceramic glaze made from ash or containing portions of ash—can be traced back to the beginnings of pottery, one of humanity’s oldest crafts.34 Since prehistoric times, all ceramics have been fired, to make them durable. A minimum firing temperature of about 900°C is required for the structure of the clay to irreversibly solidify.35 We can assume that ceramics were predominantly fired with wood until modern times. Primarily Chinese potters advanced their kilns over the centuries to achieve higher firing temperatures. Glazing with ash began around 1500 BCE, during the Shang Dynasty in China, initially by accident, when the white, glowing wood ash drifted through the kiln and fell onto the pottery. At temperatures of around 1,170°C, the ash reacted with the silicate and aluminate in the clay, forming a rough, often flowing glaze.36 As the temperature in the kiln rose, the draft of gasses from the fire opening through the firing chamber to the chimney increased, and parts of the wood ash generated during combustion were whirled through the kiln by this draft. After the firing, the potters noticed that the ceramics on the side facing the fire were often covered with glasslike coatings, sometimes more, sometimes less, depending on how exposed they were to the draft of the fire. By 1000 BCE, potters recognized that ash settling on the pieces caused these coatings and began adding ash as a glaze before placing the pots in the kiln. Ash glaze was the first glaze used in East Asia and contained only ash, clay, and water.37 The incorporation of glazing into the technical repertoire marks a significant advancement. Not only did the pots become tougher, harder, and nearly waterproof, but the addition of a colored, glasslike surface also introduced new aesthetic possibilities.38
Ceramic Glazing with Plant Ash
The composition of plant ash is highly diverse. In the Eastern pottery tradition, artists often worked with the ashes at hand and embraced their impurities as an inevitable part of life. These imperfections were not just utilized, but seamlessly integrated into the work.39 The ash itself came from various plants that were burned in specific ways.40
The chemical composition of plant ash varies depending on species, the age of the plants, the season of collection, and the location where the material grew. Generally, plant ashes can be categorized into two main types based on their composition: ash with a high calcium content, which mostly comes from wood and shrubs, and ash with a high silicate content, primarily derived from grasses and cereal plants.41 The chemical elements found in plant ash include potassium, sodium, magnesium, calcium, phosphorus, sulfur, silicon, iron, chlorine, iodine, manganese, aluminum, and bromine. In very small amounts, copper and silver are also present.42 Some plant ashes contain enough silicon dioxide to form a glass on the ceramic shard without the addition of other raw materials. However, plant ashes most primarily consist of oxides that act as fluxing agents.43 Calcium and potassium play a significant role in glazing and are present in relatively large quantities in most plant ashes.44 Trees exhibit higher concentrations of certain minerals in specific areas within their structure, such as high amounts of calcium in the bark of conifer trees.45 This calcium can be used as a primary fluxing agent in glazes.46 Substances such as potash and soda are highly alkaline and influence the melting behavior of silicon.47
Moreover, ashes serve as coloring and crystal-forming components that influence the appearance and structure of the glaze. Most ash glazes are either colorless or show shades of brown in various nuances; in reduction firing, celadon-green tones can also emerge.48 The surface can be transparent, matte, satin, or highly glossy. Among the coloring oxides present in plant ashes, iron and manganese are particularly noteworthy. Iron can be present in wood ash in amounts of up to 15 percent, while manganese in some cases, such as spruce ash, can show high values of up to 22.96 percent.49
Ash glazes and ash-fluxed glazes are valued and produced worldwide to this day. Compared to glazes with complex additives and rare raw materials, ash glazes are preferable from an ecological standpoint since they are made almost exclusively from resources that are locally available. A major challenge of using plant ashes is their varying composition, as just described. As a result, ash glaze recipes are often approximations, and consistency between different batches is difficult to achieve.
Washing ash can be a beneficial step in the production of glazes.50 All plant ashes contain soluble compounds like potash and other carbonates, sulfates, and chlorides. To avoid issues like deflocculation in glazes and burns on unprotected skin caused by some unwashed ashes that can form a fairly strong lye when dissolved in water, a certain amount of these soluble alkalis needs to be removed. The washing and sieving of ashes can be relatively time-consuming and intense, which prolongs the manufacturing process.
In summary, ash glazes offer an interesting and valuable method for ceramic production, deeply rooted in history and still practiced worldwide. Nevertheless, there are challenges to consider, especially regarding the reproducibility of results. The potential uses of tree-bark ash (industrial and others) were explored in the dissertation of Charlett Wenig and will be described within this article.
The focus here is on exploring the potential of bark ash for ceramic glazes, distinct from glass production. While a pure and transparent glaze shares similarities with glass, other glazes can differ in criteria such as strength, thermal expansion, and atomic arrangement. In ceramics, stability primarily stems from the object’s body, contrasting with the glaze’s role in protection and decoration. While conventional glass applications avoid phenomena like crystallization or cracking, these are sometimes desired and necessary for achieving specific glaze effects in ceramics.
Experimental Implementation of the Bark Ash Glaze
To explore possibilities of using bark ashes for ceramics, barks of selected tree species were burned and their chemical composition analyzed by inductively coupled plasma mass spectrometry (ICP-MS).51 The ashes were each mixed with a transparent porcelain glaze according to recipe. Hand-made porcelain bowls were glazed and then fired. The results are analyzed visually.52
Production of Bark Ash
For the first incinerations, several kilograms of tree bark were used.53 Oak (Quercus robur), spruce (Picea abies), larch (Larix decidua), pine (Pinus sylvestris), robinia (Robinia preudoacacia), and birch (Betula pendula) were used in the burning procedure.54 Tree bark was burned in a gas furnace at the University of Arts in Bremen, Germany, and at the pottery studio Müritzkeramik in Lärz, Germany. All fires were conducted under ambient pressure.
For the burning procedure, all bark samples were placed in clay pots with a volume of around ten liters. During the first firing, the barks were burned with a maximum temperature of approximately 665°C and a holding time of one hour. Since there was still too much charcoal after the first firing, the barks were burned again with a maximum temperature of approximately 1,000°C and a holding time of five and a half hours, which still resulted in an incomplete combustion of bark (fig. 1). The combustion problems in this experiment are probably caused by the function of tree bark to protect the cambium against heat and fire.55 These protective properties of bark vary widely among tree species. Decisive factors are thermal conductivity, heat transfer, moisture content, thickness, density, and chemical composition.56 Therefore, equal incineration temperatures for all bark samples are difficult to determine.57

Creation of Porcelain Bowls
As a base material for testing the glazes, porcelain bowls were produced.58 The shapes of the bowls were designed to evaluate different properties of the glazes. If the composition of the glaze in relation to the firing temperature is good, the glaze layer will have an equal thickness in the center of the bowl and on the walls. Otherwise, the glaze will run down the walls, forming a thicker layer at the center of the bowl, which can significantly change the coloration.59
Production of a Bark-Ash Glaze
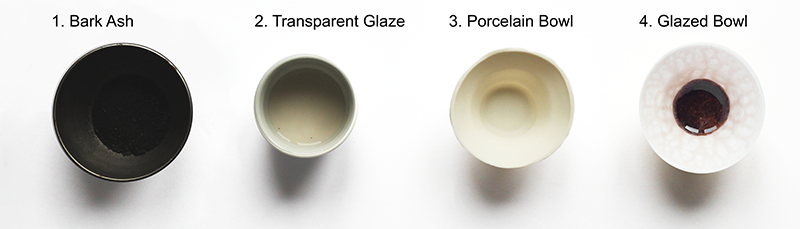
For the production of a bark-ash glaze, filtered bark ash (fig. 2.1) was added to the transparent glazes (fig. 2.2) before the firing process. Because of the limited quantities of bark ash, the ash was not washed.60 The recipe for the glaze is given in table 1.
In these experiments, bark ashes of oak (Quercus robur), spruce (Picea abies), larch (Larix decidua), pine (Pinus sylvestris), robinia (Robinia preudoacacia), and birch (Betula pendula) were used. As a further test series, an alternative recipe for the production of matte glaze was used (table 2).
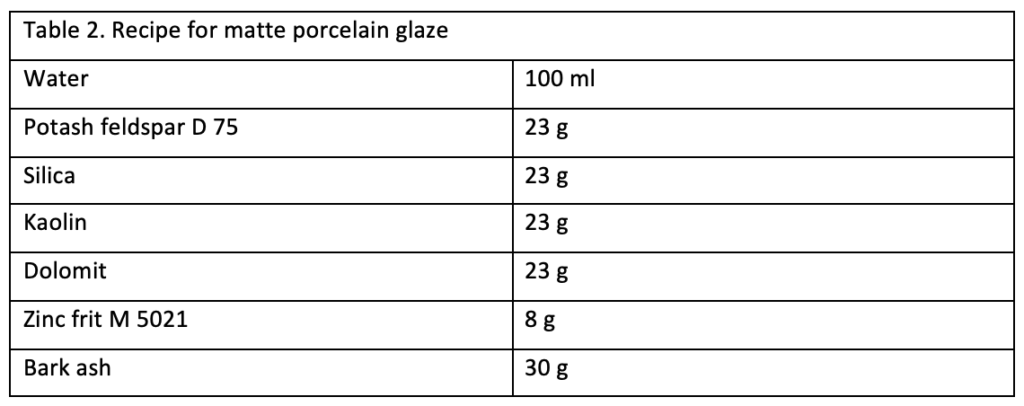

The first results showed that all transparent glazes were colored in combination with bark ash (fig. 3). The colorations ranged from yellow to brown to blue-green tones. Most of the glaze moved to and accumulated at the center of the bowl, where a thick layer of glaze was formed. All glazes bonded with the ceramic body. A few bowls had a transparent sheen on the interior walls, while others were matte. In the following text, selected results will be discussed.

The larch glaze, as depicted in figure 4.1, presented a completely covered, glossy beige finish. This glaze is fully closed, with colors ranging from beige to blue and purple, depending on layer thickness. An especially interesting outcome was observed with the spruce ash glaze, shown in figure 4.2. The spruce glazes coated the inside walls of the bowl with a thin layer that netted to form thicker strands, resulting in a meshlike design that cascades to the base of the bowl, creating a dense pool of deep-brown glaze. Birch glaze, shown in figure 4.3, displays a closed, intensely rose color, which could be seen especially in thicker layers at the bottom of the bowl. These colors are also found in the production of antique glass, caused by the oxides of copper, iron, and manganese.61
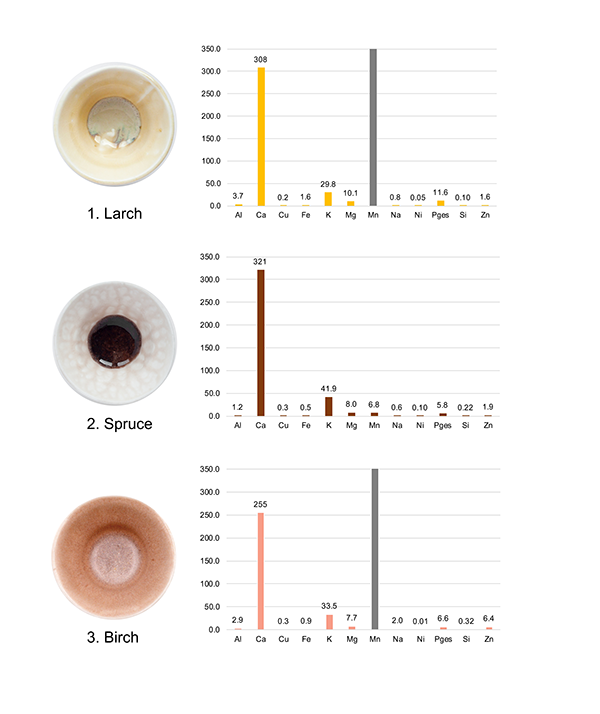
To gain a deeper understanding of the chemical composition of bark ashes and how it influences the development of glaze colors, the distribution of mineral constituents in different barks was examined. This was achieved through ICP-MS measurements, as depicted in figure 5. Predominantly, calcium emerged as the main element present in all bark ashes, with potassium coming in next.62 A reason for the high calcium content is the presence of calcium oxalate crystals, which are frequently located in various parts of bark.63 Besides calcium and potassium, other elements, primarily metals such as copper, iron, magnesium, and natrium, as well as silica and phosphorus, were detected in smaller amounts. These findings align with previous research on the chemical composition of bark ash.64 Interestingly, manganese, which is typically only a trace element in bark, was found in high concentrations in both larch ash and birch ash (figs. 5.1 and 5.3).
The chemical composition of bark depends on the type of tree and the soil it grows in. Numerous instances in the literature indicate that unusually high quantities of manganese in plants likely originate from the nearby soil, leading to variations in the chemical composition of each tree based on its growth location.65 However, since the measured ICP-MS values are similar to those found in the literature, the general composition of individual bark types can be estimated.66
How does the composition influence the glazing outcome? While the bowl covered with the pure glaze possesses a clear and transparent surface, the spruce ash glaze shows a strong deep-brown coloration. This color forms a weblike pattern, extending from the vessel’s walls to its center, as depicted in figure 4.2. Such an effect is known to occur in glazes with high concentrations of calcium oxide.67 We found that spruce ash contains a large proportion of calcium (see fig. 5).
In contrast, the birch-bark ash glaze developed a strong matte finish with a rose color, as illustrated in figure 4.3. The rose color observed in the glaze may be a result of the connection between boron (sourced from the primary glaze recipe) and the high manganese content. According to Lehnhäuser, such a combination can manifest as a violet shade in glazes.68 The transition from a shiny to a matte finish can be caused by various factors. Considering that the foundational glaze is glossy, the matte texture of the ash glaze could be a result of a problem during the firing stage, such as remnants of charcoal in the glaze, which can lead to gas release during the firing phase. Alternatively, the quite high zinc content in birch ash can also promote matting and crystallization.69
The larch bark developed a uniform light-beige glaze. This glaze tends to accumulate at the bottom, morphing into a thicker bluish-purple layer, as shown in figure 4.1. Robert Tichane asserts that manganese oxidation may account for the amber tint of many oxidized ash glazes.70 In this case the high manganese content is more likely to be the reason for the beige color than the iron content. The bluish color might be caused by the boron in the transparent glaze.71
The results obtained for the transparent glaze (table 1) from the first test series (see fig. 4) were replicated in a second series (fig. 6). For all bark ashes, differences were observed in the color intensity. Additionally, a transition from matte to glossy was observed in the birch glaze.

The shift in color of the larch-bark ash glaze can be caused by a thicker layer of glaze and the use of a different kiln, which might be responsible for the amplified color intensity. In the case of the birch-ash glazing experiments, there were changes in finish, from matte to glossy, and minor changes in color intensity. Conversely, the glazing results for spruce vary significantly, transitioning from a deep brown in the first test series to a light beige in the second. This difference might come from the second firing in a different kiln and from alterations in the concentration and distribution of chemical components between the two test series. The variance in the distribution of individual glazing components could likely stem from variations in the mixing of the glazes used in the first and second rounds of testing. Glazes are usually not solutions but suspensions. Therefore, it is important to mix and sieve them carefully before use. Since all glaze tests were performed manually, there is a potential for inconsistencies in the blending process across the two test series. This could lead to the color change in the spruce glazes between the first and the second series.
For further investigations, the glaze experiments were extended by an additional recipe for a colorless matte glaze (see table 2). The main goal of this test was the reevaluation of the coloration. Since both glazes are colorless before the addition of the ash, it is expected that they will exhibit the same color spectrum. An advantage of the selected matte glaze recipe is that it contains no boron. This eliminates potential color variations due to such additives.
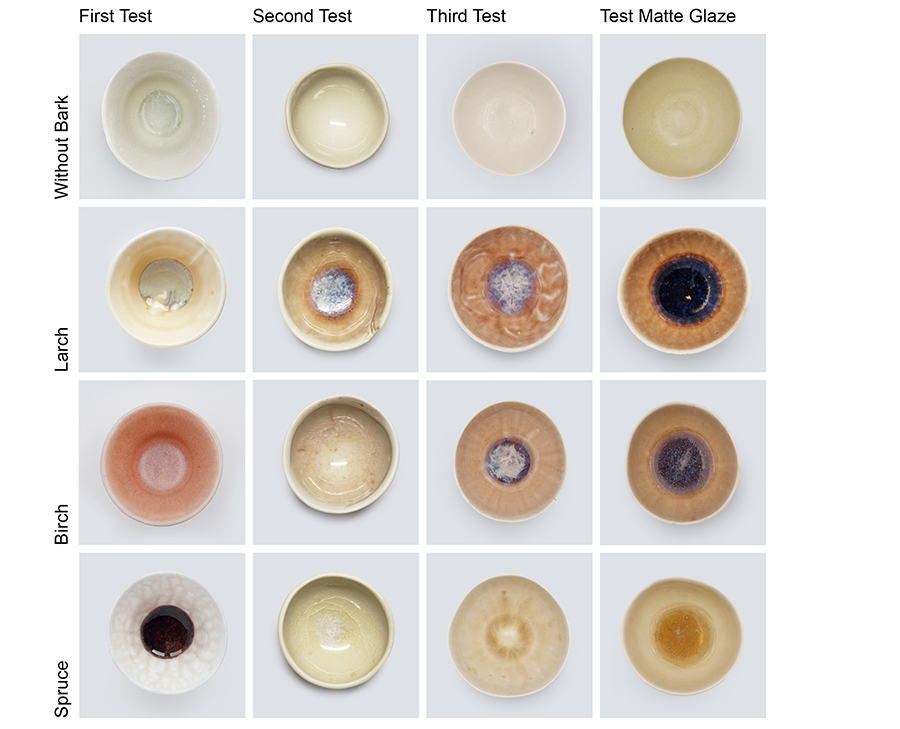
Results presented in figure 7 provide an overview of all bark glazing samples. In the second and third tests using the transparent glaze containing boron, variations in color and saturation were observed. The third outcome of the larch-ash sample displayed a glossy glaze, appearing slightly darker this time, yet still within the same reddish-brown color spectrum on the outer walls and bluish-purple color at the bowl’s center. Birch glaze shared a color range closer to the results from the larch ash. Spruce showed a transparent, slightly yellowish glaze without any blue color traces in the center.
The fourth column of figure 7 displays the results of the matte glaze (see table 2). In comparison with the other transparent glaze (see table 1), this one exhibited a smooth matte finish, yet lacked a neutral color, revealing an inherent light-beige tint. The result for the larch ash showed a glossy surface and a darker color this time. The outer walls are covered with a reddish-brown glaze, and the center of the bowl has a thick, shiny, red-brown glaze surface. Birch produced a glossy coloring in deep red-brown. The glaze showed a low viscosity and a lot of glaze run at the bowl’s center, resulting in a color spectrum ranging between purple and brown with white dots and covered by a continuous yet irregular surface. The combination of this matte glaze with spruce led to a soft beige glaze on the walls and a yellow glaze at the center. The surface of the spruce glaze developed a craquelure.
The variability of all results shows the precision required for this crafting process and indicates that a certain degree of variability in color and surface quality cannot be avoided. Beyond the described challenges in glaze outcomes, difficulties appeared in producing bark ash for these test series. Incompletely burned portions of charcoal in the ash—weighing more than the fully burned ash would weigh—lead to inaccurately measured ash quantities. Such discrepancies can alter the color outcome and probably lead to significantly lighter colors. Furthermore, charcoal fragments could be unevenly distributed within the glaze, causing discolorations. Such issues might not play a role in industrially produced bark ashes, since they are burned for a longer time.
For the experiments, 12–14 percent ash relative to the total glaze volume was used. Conventionally, for ash glazes, the standard quantity of plant-based ash is around 30 percent by weight, as stated by Tichane in 1998. Tichane also mentions the feasibility of high-ash glazes ranging from 30 percent to 60 percent, and even up to 100 percent.72
Discussion
To conclude the results of the glazing experiments, the high calcium content in bark suggests that tree-bark ash could act as a natural flux in glazes. The observed color spectrum, ranging from yellow to brown and red-purple shades, is likely influenced by the oxides of elements like copper, iron, and manganese. If this diversity in colors is unwanted, further research may be needed to achieve more homogenous results.
The present study aims to provide a first insight into the use and effects of bark ash in glazes, giving new life to a leftover material that would otherwise represent the “dead end” of a series of industrial processes. The focus was on the observation of alteration in color in a broad spectrum by incorporating bark ash into a glaze mixture. Future extensive and detailed experimental series are needed to surpass the current general and qualitative perspective. These studies could include the exploration of impacts of the added bark ashes on physical properties such as the coefficient of thermal expansion, glaze stresses, and melting point. Additionally, investigating the effect of the typically high calcium content in the ashes on glaze properties and crystallization would be relevant. The possibilities of tree-bark glazes have not yet been exploited.
Designing for Sustainability
In the evolving landscape of product design and manufacturing, several key concepts are shaping our perceptions and expectations. “Zero waste” has emerged as both an environmental imperative and a source of added value. It challenges traditional notions of waste disposal by emphasizing sustainability and resource optimization. This concept encourages us to rethink our approach to materials and their impact on the environment.
In this context, the dichotomy between one-of-a-kind, unique pieces and mass-produced pieces becomes increasingly relevant. As industrialization has progressed, it has sparked a shift in values. The relentless pursuit of mass production prompts us to question what is truly valuable in our products and possessions. Are we sacrificing uniqueness and cultural significance for the sake of mass-production efficiency?
Visually translating the complex chemical compositions and significant individual variations found in tree bark into ceramics brings authenticity to the forefront, highlighting the uniqueness of different bark ashes and the value of their inherent qualities. Design is used to make research results transparent and tangible for a larger audience and to highlight materials as active participants in the design process.
At the intersection of these concepts lies the science of glaze chemistry, which has evolved significantly over the past decades. The ceramics industry has developed glazes that are functional, sanitary, long-lasting, and reproducible in their surface structure and color. These glazes are made from pure sources and processed minerals acquired around the world.73
However, the industrial handling and sourcing of glaze components greatly differ from the original process of glaze production. While (ash) glazes were once crafted from local components, today the collection and production of glazes are geographically distant processes. Industrial glaze often lacks cultural context and is perceived as replaceable. Examining the diversity of plant ash and analyzing the transformation after firing the glaze is a craft in itself.
The use of bark ash is motivated by the desire to utilize every leftover of the natural resource, bark, thus aligning with the “zero waste” concept. Using bark ash could reintroduce a material that is currently perceived as a “special craft material” but produced on industrial scales back into widespread use. It is essential to view the variability of the glazes not as a problem but as an opportunity to appreciate their value—in their uniqueness and the long-standing tradition that links them with human culture. Instead of regarding these properties as weaknesses to be overcome, they should be recognized as natural boundaries that inform and shape the design process. The knowledge required for processing biomaterials is therefore more multifaceted and complex than that needed for many synthetic materials. Designing or manufacturing with tree-bark ash, for example, is undoubtedly not controllable to the same extent as we are used to with current (synthetic) materials. However, the variability of a material like tree bark also holds great potential. Such variable raw materials have long accompanied humans through our cultural evolution, refined in craftsmanship, and scientifically analyzed for their intrinsic processes and their interactions with the environment. Combining methodologies and knowledge from science, humanities, history, and crafts can build a comprehensive research practice. Merging these diverse fields offers shared inspiration, sustainable outcomes, and new research questions. This cross-disciplinary method could shift our perspective on current waste products, redefining their value and function in society. It encourages us to explore the intrinsic value of materials, the significance of cultural heritage, and the potential for a more sustainable and authentic approach to design and manufacturing.
Charlett Wenig
Charlett Wenig is an interdisciplinary material researcher and industrial designer in the research group Adaptive Fibrous Materials at the Max Planck Institute of Colloids and Interfaces, Potsdam, Germany. She is interested in local biomaterials and their processing possibilities.
Michaela Eder
Michaela Eder is interested in how plant materials such as wood, bark, or seedpods function for the organism and when applied as materials. She is professor of wood science and functionalization at the TUM School of Life Sciences, Freising, Germany.
Dirk Friese
Dirk Friese is a ceramist and artist based near Berlin. Before dedicating himself to art, he worked as an IT system administrator and occupational therapist. He currently leads Atelier Saralya, Kotzen, Germany.
Johanna Hehemeyer-Cürten
Johanna Hehemeyer-Cürten is a fashion and material designer and a PhD student in the research group Adaptive Fibrous Materials at the Max Planck Institute of Colloids and Interfaces, Potsdam, Germany. She is interested in local pine bark and its processing possibilities.
The authors’ addresses are as follows: CW, JH-C, and ME: Department of Biomaterials, Max Planck Institute of Colloids and Interfaces, Am Mühlenberg 1, 14476 Potsdam, Germany; DF: Atelier Saralya, Parkstraße 1, 14715 Kotzen, Germany.
In this study, CW, JH-C, and DF designed and made the bark-ash glazes and porcelain bowls. CW wrote the first draft of the manuscript, with text contributions related to the chemical composition of glazes and the outcome of the bark-ash glazes from DF. The figures were created by CW and JH-C. ME initiated the multidisciplinary collaboration and was involved in numerous scientific discussions related to the project. All authors discussed, commented on, and approved the manuscript.
The authors declare that they have no competing interests. CW, JH-C, and ME acknowledge the support of the Cluster of Excellence “Matters of Activity: Image Space Material” funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany’s Excellence Strategy—EXC 2025-390648296 and the Max-Planck Society.
We thank the foresters Marko Robakowski and Uwe Peschke and Furnierwerk Merkscha GmbH for providing tree bark for the glazing experiment. The recipe for the transparent porcelain glaze (borate glaze) was kindly provided by the ceramic workshop supervisor Ute Fischer (University of the Arts Bremen).
- 1 Charlett Wenig, John W. C. Dunlop, Johanna Hehemeyer-Cürten, Friedrich J. Reppe, Nils Horbelt, Karin Krauthausen, Peter Fratzl, and Michaela Eder, “Advanced Materials Design Based on Waste Wood and Bark,” Philosophical Transactions of the Royal Society A 379, no. 2206 (2021): https://doi.org/10.1098/rsta.2020.0345.
- 2 Within this research scope, soft-paste porcelain and corresponding glazes are utilized and fired at a temperature within the stoneware range.
- 3 Lucas Stephens, Dorian Fuller, Nicole Boivin, Torben Rick, Nicolas Gauthier, Andrea Kay, Ben Marwick, Chelsey Geralda Armstrong, C. Michael Barton, and Tim Denham, “Archaeological Assessment Reveals Earth’s Early Transformation through Land Use,” Science 365, no. 6456 (2019): 897–902.
- 4 Yinon M. Bar-On, Rob Phillips, and Ron Milo, “The Biomass Distribution on Earth,” Proceedings of the National Academy of Sciences 115, no. 25 (2018): 6506–11.
- 5 Beat Schneider, Design-eine Einführung: Entwurf im sozialen, kulturellen und wirtschaftlichen Kontext (Berlin: De Gruyter, 2005): 94–95.
- 6 Schneider, Design-eine Einführung.
- 7 Bar-On et al., “Biomass Distribution on Earth.”
- 8 Destatis, “Exporte von Rohholz im Jahr 2020 um 42,6 % gestiegen,” 2021, https://www.destatis.de/DE/Presse/Pressemitteilungen/2021/05/PD21_N031_51.html.
- 9 Udo Mantau, “Holzrohstoffbilanz Deutschland: Entwicklungen und Szenarien des Holzaufkommens und der Holzverwendung von 1987 bis 2015” (Hamburg, 2012), 65, https://epub.sub.uni-hamburg.de/epub/volltexte/2013/23574/.
- 10 Wenig et al., “Advanced Materials.”
- 11 John M. Harkin, Bark and Its Possible Uses (Madison: Forest Products Laboratory, US Forest Service, 1971), 1–25.
- 12 Ing Ralf Wollenberg and Dipl-Ing Christian Warnecke, “Neue Einsatzgebiete für Rinden durch Produktentwicklung” (Dissertation Maschinenbau, Verfahrens- und Energietechnik, Technischen Universität Bergakademie, Freiberg, 2005).
- 13 Shanghuan Feng, Shuna Cheng, Zhongshun Yuan, Mathew Leitch, and Chunbao Charles Xu, “Valorization of Bark for Chemicals and Materials: A Review,” Renewable and Sustainable Energy Reviews 26 (2013): 560–78.
- 14 Paul R. B. Kozowyk, Marie Soressi, Diederick Pomstra, and Geeske H. J. Langejans, “Experimental Methods for the Palaeolithic Dry Distillation of Birch Bark: Implications for the Origin and Development of Neandertal Adhesive Technology,” Scientific Reports 7, no. 1 (2017): 8033.
- 15 Marion Kite and Roy Thomson, Conservation of Leather and Related Materials (Abingdon: Routledge, 2006): 82–97, 185.
- 16 Wenig et al., “Advanced Materials.” All examples are taken from the collection of the British Museum (https://www.britishmuseum.org/collection, last accessed March 6, 2021). See museum numbers Oc.4061 (netted bag made of bark-fiber cord, made by aboriginal Australian, nineteenth century), 2016,8030.1 (bag made of plaited strips of birch bark from Karelia in Russia/former Finland, 1900–40), Am1928,-.92 (mat made of birch bark, with quill works and feathers, from North America, bought 1928), 2011,8036.35 (pot scourer in the form of a ball, made of curled birch-bark strips, from Finland, 2011), Am1949,22.170 (scroll made of birch bark, with zoomorphic drawings, made by the Ojibwa, Minnesota, bought 1949), and Oc1894,0814.4 (oblong sheet of barkcloth with geometrical designs from Taveuni in Fiji/Oceania, nineteenth century). For barkcloth around the world, see Dawei Li, Wei Wang, Feng Tian, Wei Liao, and Christopher J. Bae, “The Oldest Bark Cloth Beater in Southern China (Dingmo, Bubing basin, Guangxi),” Quaternary International 354 (2014): 184–89, https://doi.org/10.1016/j.quaint.2014.06.062; Chi-Shan Chang, Hsiao-Lei Liu, Ximena Moncada, Andrea Seelenfreund, Daniela Seelenfreund, and Kuo-Fang Chung, “A Holistic Picture of Austronesian Migrations Revealed by Phylogeography of Pacific Paper Mulberry,” Proceedings of the National Academy of Sciences 112, no. 44 (2015): 13537–42, https://doi.org/10.1073/pnas.1503205112; Judith Cameron, “Trans-Oceanic Transfer of Bark-Cloth Technology from South China-Southeast Asia to Mesoamerica,” Islands of Inquiry: Colonisation, Seafaring and the Archaeology of Maritime Landscapes 29 (2008): 203–10; and Samson Rwawiire, George William Luggya, and Blanka Tomkova, “Morphology, Thermal, and Mechanical Characterization of Bark Cloth from Ficus natalensis,” ISRN Textiles 2013 (2013): 925198, https://doi.org/10.1155/2013/925198.
- 17 E. T. Adney and H. I. Chapelle, Bark Canoes & Skin Boats of North America (Washington, DC: Smithsonian Institute, 1964), 67.
- 18 Hiltraud Ast and Georg Winner, “Historische Holzverwendung und Waldnutzung in der Schneebergregion: Rindennutzung,” Universität für Bodenkultur, Institut für Holztechnologie und Nachwachsende Rohstoffe (2011), last accessed February 28, 2024, https://www.yumpu.com/user/holzverwendung.boku.ac.at.
- 19 Harkin, Bark.
- 20 Harkin, Bark.
- 21 A. Schuster, N. Ortmayr, G. J. Oostingh, and B. Stelzhammer, “Compounds Extracted from Larch, Birch Bark, Douglas Fir, and Alder Woods with Four Different Solvents: Effects on Five Skin-Related Microbes,” BioResources 15, no. 2 (2020): 3368–81; Y. Yazaki “Utilization of Flavonoid Compounds from Bark and Wood: A Review,” Natural Product Communications 10, no. 3 (2015): https://doi.org/1934578X1501000333.
- 22 Wenig et al., “Advanced Materials.”
- 23 Feng et al., “Valorization.”
- 24 Sumrerng Rukzon and Prinya Chindaprasirt, “Use of Rice Husk-Bark Ash in Producing Self-Compacting Concrete,” Advances in Civil Engineering 2014 (2014): 1–6.
- 25 Roger M. Rowell, Roger Pettersen, James S. Han, Jeffrey S. Rowell, and Mandla A. Tshabalala, “Cell Wall Chemistry,” in Handbook of Wood Chemistry and Wood Composites, ed. Roger M. Rowell (Boca Raton, FL: CRC Press, 2005), 43–47; Dietrich Fengel and Gerd Wegener, Wood: Chemistry, Ultrastructure, Reactions (Berlin: De Gruyter, 2011), 217; E. F. Kurth, “The Chemical Composition of Barks,” Chemical Reviews 40, no. 1 (1947): 33–49.
- 26 Matthew Canti and Jacques Elie Brochier, “Plant Ash,” in Archaeological Soil and Sediment Micromorphology, ed. Cristiano Nicosia and Georges Stoops (London: Wiley, 2017), 147–54.
- 27 Richard W. Bryers, “Fireside Slagging, Fouling, and High-Temperature Corrosion of Heat-Transfer Surface Due to Impurities in Steam-Raising Fuels,” Progress in Energy and Combustion Science 22, no. 1 (1996): 29–120.
- 28 Fengel and Wegener, Wood.
- 29 Rukzon and Chindaprasirt, “Use of Rice”; César Pérez-Cruzado, Fernando Solla-Gullón, Agustín Merino, and Roque Rodríguez-Soalleiro, “Analysis of Growth and Nutrition of a Young Castanea x coudercii Plantation after Application of Wood-Bark Ash,” European Journal of Forest Research 130, no. 2 (2011): 209–17.
- 30 Ivana Angelini, Bernard Gratuze, and Gilberto Artioli, “Glass and Other Vitreous Materials through History,” EMU Notes Mineral 20 (2019): 87–150; Nadine Schibille, Islamic Glass in the Making: Chronological and Geographical Dimensions (Leuven: Leuven University Press, 2022), 125.
- 31 Youssef Barkoudah and Julian Henderson, “Plant Ashes from Syria and the Manufacture of Ancient Glass: Ethnographic and Scientific Aspects,” Journal of Glass Studies 48 (2006): 297–321, http://www.jstor.org/stable/24191157; Joaquín Angel Ortuño et al., “Halophytes in Arts and Crafts: Ethnobotany of Glassmaking,” in Handbook of Halophytes: From Molecules to Ecosystems towards Biosaline Agriculture (Cham: Springer International, 2021), 2675–706.
- 32 Caroline M. Jackson and Jim Smedley, “Theophilus and the Use of Beech Ash as a Glassmaking Alkali 1,” Archaeology, History and Science (2016): 117–30.
- 33 C. Barry Carter and M. Grant Norton, Ceramic Materials: Science and Engineering, vol. 716 (New York: Springer, 2007), 17–22.
- 34 Wolf Matthes, Keramische Glasuren 2, Neue Erkenntnisse—Neue und alte bewährte Rezepturen (Koblenz: Hanusch Verlag, 2012), 21–34.
- 35 Gustav Weiss, Abenteuer Erde und Feuer: das ist Keramik (Bern: Haupt, 2000), 56.
- 36 Robert Tichane, Ash Glazes (Iola, WI: Krause Publications, 1998), 5–40.
- 37 Phil Rogers, Ash Glazes (Philadelphia: University of Pennsylvania Press, 2003), 10–22, 22–24, 42.
- 38 Rogers, Ash Glazes.
- 39 Rogers, Ash Glazes.
- 40 Michail A. Bezborodov, Chemie und Technologie der antiken und mittelalterlichen Gläser (Mainz: Zabern, 1975), 46–55.
- 41 Matthes, Keramische Glasuren 2; Miranda Forrest, Natural Glazes: Collecting and Making (London: Herbert Press, 2018), 10ff.
- 42 Rogers, Ash Glazes.
- 43 Daniel Rhodes and Robin Hopper, Ton und Glasur: Verstehen und anwenden (Lahnstein: Hanusch, 2006), 293.
- 44 Matthes, Keramische Glasuren.
- 45 J. W. Hudgins, Trygve Krekling, and Vincent R. Franceschi, “Distribution of Calcium Oxalate Crystals in the Secondary Phloem of Conifers: A Constitutive Defense Mechanism?,” New Phytologist 159, no. 3 (2003): 677–90.
- 46 Tichane, Ash Glazes.
- 47 Rogers, Ash Glazes.
- 48 Jan-Erik Nilsson, “Celadon,” in Gotheborg.com, 2019, https://www.gotheborg.com/glossary/celadon.shtml.
- 49 Rogers, Ash Glazes.
- 50 Tichane, Ash Glazes.
- 51 ICP-MS is an analytical technique used for the detection of trace metals and some nonmetals at relatively low concentrations.
- 52 The experiments are described in Charlett Wenig, “Sustainable Tree Bark Objects by Combining Science and Design” (Dissertation Prozesswissenschaften, Technische Universität, Berlin 2023). The inductively coupled plasma measurements are available from Edmond—the Open Research Data Repository of the Max Planck Society. The glazing samples are stored at the Max Planck Institute of Colloids and Interfaces, Am Mühlenberg 1, 14476 Potsdam.
- 53 Industrial bark waste from the sawmills Furnierwerk Merkscha GmbH in Gratwein and Furnierwerk Prignitz GmbH und CoKg in Pritzwalk and manually peeled bark was used.
- 54Before incineration, the bark was stored under lab conditions (approximately 22°C and approximately 50 percent relative humidity) until further processing.
- 55 The cambium is the growing part of the stem. It produces new bark cells to the outside and new wood cells to the inside. Andreas Bär and Stefan Mayr, “Bark Insulation: Ten Central Alpine Tree Species Compared,” Forest Ecology and Management 474 (2020): 118361, https://doi.org/10.1016/j.foreco.2020.118361; A. Kupferschmid, “Holzkunde II Teil 4, Rindenkunde und Rindenverwertung,” Research Collection ETH Zürich (2001): https://doi.org/10.3929/ethz-a-004536640.
- 56 Juli G. Pausas, “Bark Thickness and Fire Regime,” Functional Ecology 29, no. 3 (2015): 315–27; Karl W. Spalt and Williamam E. Reifsnyder, Bark Characteristics and Fire Resistance: A Literature Survey, Occasional Paper 193 (USDA Forest Service, Southern Forest Experiment Station, 1962), 4–16.
- 57 For comparable results, we decided not to burn each tree bark individually.
- 58 The porcelain bowls were produced at the University of Arts in Bremen.
- 59 Wolf E. Matthes, Keramische Glasuren: Grundlagen, Eigenschaften, Rezepte, Anwendungen (Cologne: Rudolf Müller, 1985).
- 60 Limited quantities prompted the decision not to wash the ashes before processing in the described test series assessing the potential for colored glaze from tree bark. Future research may explore differences with larger sample rows with washed bark samples.
- 61 Tichane, Ash Glazes; Matthes, Keramische Glasuren.
- 62 Bryers, “Fireside Slagging.”
- 63 Calcium oxalate crystals are located in sieve cells and longitudinal parenchyma in the phloem and phelloderm. See Ray F. Evert, Esau’s Plant Anatomy: Meristems, Cells, and Tissues of the Plant Body; Their Structure, Function, and Development (London: Wiley, 2006), 409–10.
- 64 Fengel and Wegener, Wood; Isabel Miranda, Jorge Gominho, Inês Mirra, and Helena Pereira, “Chemical Characterization of Barks from Picea abies and Pinus sylvestris after Fractioning into Different Particle Sizes,” Industrial Crops and Products 36, no. 1 (2012): 395–400, https://doi.org/10.1016/j.indcrop.2011.10.035; K. E. Saarela, L. Harju, J. O. Lill, S. J. Heselius, J. Rajander, and A. Lindroos, “Quantitative Elemental Analysis of Dry-Ashed Bark and Wood Samples of Birch, Spruce and Pine from South-Western Finland Using PIXE,” Acta Academiae Aboensis, Ser B 65, no. 4 (2005): 8, 23–24.
- 65 Jackson and Smedley, “Theophilus.”
- 66 Fengel and Wegener, Wood; Miranda et al., “Chemical Characterization of Barks”; Saarela et al., “Quantitative Elemental Analysis.”
- 67 Matthes, Keramische Glasuren.
- 68 Werner Lehnhäuser, Glasuren und ihre Farben (Olten: Knapp, 1985), 134–35.
- 69 Lehnhäuser.
- 70 Tichane, Ash Glazes.
- 71 Lehnhäuser, Glasuren. Boron, which has pronounced melting and bonding properties, increases the color adhesion but can also cause a blue tint.
- 72 Tichane, Ash Glazes, 5–40.
- 73 Forrest, Natural Glazes.

